Xray views

X-rays are part of the electromagnetic spectrum, with wavelengths shorter than visible light. Different applications use different parts of the X-ray spectrum.
X-rays make up X-radiation, a form ofelectromagnetic radiation. Most X-rays have awavelength ranging from 0.01 to 10nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz (3×1016Hz to 3×1019 Hz) and energies in the range 100 eV to 100 keV. X-ray wavelengths are shorter than those of UV rays and typically longer than those of gamma rays. In many languages, X-radiation is referred to with terms meaning Röntgen radiation, after the German scientist Wilhelm Röntgen,[1] who usually is credited as its discoverer, and who had named it X-radiation to signify an unknown type of radiation.[2] Spelling of X-ray(s) in the English language includes the variants x-ray(s), xray(s), and X ray(s).[3]
History
Discovery
German physicist Wilhelm Röntgen is usually credited as the discoverer of X-rays in 1895, because he was the first to systematically study them, though he is not the first to have observed their effects. He is also the one who gave them the name "X-rays" (signifying an unknown quantity[4]) though many others referred to these as "Röntgen rays" (and the associated X-ray radiograms as, "Röntgenograms") for several decades after their discovery and even to this day in some languages, including Röntgen's nativeGerman.
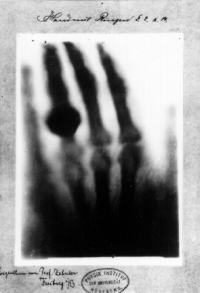
Hand mit Ringen (Hand with Rings): print of Wilhelm Röntgen's first "medical" X-ray, of his wife's hand, taken on 22 December 1895 and presented to Ludwig Zehnderof the Physik Institut, University of Freiburg, on 1 January 1896[5][6]
X-rays were found emanating from Crookes tubes, experimental discharge tubes invented around 1875, by scientists investigating thecathode rays, that is energetic electronbeams, that were first created in the tubes. Crookes tubes created free electrons byionization of the residual air in the tube by a high DC voltage of anywhere between a fewkilovolts and 100 kV. This voltage accelerated the electrons coming from the cathode to a high enough velocity that they created X-rays when they struck the anode or the glass wall of the tube. Many of the early Crookes tubes undoubtedly radiated X-rays, because early researchers noticed effects that were attributable to them, as detailed below. Wilhelm Röntgen was the first to systematically study them, in 1895.[7]
The discovery of X-rays stimulated a veritable sensation. Röntgen's biographer Otto Glasser estimated that, in 1896 alone, as many as 49 essays and 1044 articles about the new rays were published.[8] This was probably a conservative estimate, if one considers that nearly every paper around the world extensively reported about the new discovery, with a magazine such as Science dedicating as many as 23 articles to it in that year alone.[9] Sensationalist reactions to the new discovery included publications linking the new kind of rays to occult and paranormal theories, such as telepathy.[10][11]
Early research
The earliest known experimenter with X-rays was actuary William Morgan. In 1785 he presented a paper to the Royal Society of London describing the effects of passing electrical currents through a partially evacuated glass tube, the first X-ray tube.[12]This work was further extended by Humphry Davy and his assistant Michael Faraday.
In 1876, Eugen Goldstein proved that they came from the cathode, and named themcathode rays (Kathodenstrahlen).[13] Both William Crookes (in the 1880s)[14] and German physicist Johann Hittorf,[15] a co-inventor and early researcher of the Crookes tube, found that paper wrapped photographic plates placed near the tube became unaccountably fogged or flawed by shadows, although they had not been exposed to light. Neither found the cause nor investigated this effect.
In 1877 Ukrainian-born Ivan Pulyui, a lecturer in experimental physics at the University of Vienna, constructed various designs ofvacuum discharge tube to investigate their properties.[16] He continued his investigations when appointed professor at the Prague Polytechnic and in 1886 he found that sealed photographic plates became dark when exposed to the emanations from the tubes. Early in 1896, just a few weeks after Röntgenpublished his first X-ray photograph, Pulyui published high-quality X-ray images in journals in Paris and London.[16] Although Pulyui had studied with Röntgen at theUniversity of Strasbourg in the years 1873–75, his biographer Gaida (1997) asserts that his subsequent research was conducted independently.[16]

Taking an X-ray image with early Crookes tubeapparatus, late 1800s. The Crookes tube is visible in center. The standing man is viewing his hand with afluoroscope screen. The seated man is taking aradiograph of his hand by placing it on a photographic plate. No precautions against radiation exposure are taken; its hazards were not known at the time.
X-rays were generated and detected byFernando Sanford (1854–1948), the foundation Professor of Physics at Stanford University, in 1891. From 1886 to 1888 he had studied in the Hermann Helmholtz laboratory in Berlin, where he became familiar with the cathode rays generated in vacuum tubes when a voltage was applied across separate electrodes, as previously studied by Heinrich Hertz and Philipp Lenard. His letter of January 6, 1893 (describing his discovery as "electric photography") to The Physical Review was duly published and an article entitled Without Lens or Light, Photographs Taken With Plate and Object in Darkness appeared in the San Francisco Examiner.[17]
Starting in 1888, Philipp Lenard, a student of Heinrich Hertz, conducted experiments to see whether cathode rays could pass out of the Crookes tube into the air. He built a Crookes tube (later called a "Lenard tube") with a "window" in the end made of thin aluminum, facing the cathode so the cathode rays would strike it. He found that something came through, that would expose photographic plates and cause fluorescence. He measured the penetrating power of these rays through various materials. It has been suggested that at least some of these "Lenard rays" were actually X-rays.[18]
Hermann von Helmholtz formulated mathematical equations for X-rays. He postulated a dispersion theory before Röntgen made his discovery and announcement. It was formed on the basis of the electromagnetic theory of light.[19]However, he did not work with actual X-rays.
In 1894 Nikola Tesla noticed damaged film in his lab that seemed to be associated with Crookes tube experiments and began investigating this radiant energy of "invisible" kinds.[20][21] After Röntgen identified the X-ray Tesla began making X-ray images of his own using high voltages and tubes of his own design,[22] as well as Crookes tubes.
Wilhelm Röntgen
1896 plaque published in "Nouvelle Iconographie de la Salpetrière", a medical journal. In the left a hand deformity, in the right same hand seen using radiography. The authors designated the technique as Röntgen photography.
On November 8, 1895, German physics professor Wilhelm Röntgen stumbled on X-rays while experimenting with Lenard andCrookes tubes and began studying them. He wrote an initial report "On a new kind of ray: A preliminary communication" and on December 28, 1895 submitted it toWürzburg's Physical-Medical Society journal.[23] This was the first paper written on X-rays. Röntgen referred to the radiation as "X", to indicate that it was an unknown type of radiation. The name stuck, although (over Röntgen's great objections) many of his colleagues suggested calling them Röntgen rays. They are still referred to as such in many languages, including German, Hungarian, Danish, Polish, Swedish, Finnish, Estonian, Russian, Japanese, Dutch, and Norwegian. Röntgen received the first Nobel Prize in Physics for his discovery.[24]
There are conflicting accounts of his discovery because Röntgen had his lab notes burned after his death, but this is a likely reconstruction by his biographers:[25][26]Röntgen was investigating cathode rays from a Crookes tube which he had wrapped in black cardboard so that the visible light from the tube would not interfere, using afluorescent screen painted with bariumplatinocyanide. He noticed a faint green glow from the screen, about 1 meter away. Röntgen realized some invisible rays coming from the tube were passing through the cardboard to make the screen glow. He found they could also pass through books and papers on his desk. Röntgen threw himself into investigating these unknown rays systematically. Two months after his initial discovery, he published his paper.[citation needed]
Röntgen discovered their medical use when he made a picture of his wife's hand on a photographic plate formed due to X-rays. The photograph of his wife's hand was the first photograph of a human body part using X-rays. When she saw the picture, she said "I have seen my death."[27]
Advances in radiology
In 1895, Thomas Edison investigated materials' ability to fluoresce when exposed to X-rays, and found that calcium tungstatewas the most effective substance. Around March 1896, the fluoroscope he developed became the standard for medical X-ray examinations. Nevertheless, Edison dropped X-ray research around 1903, even before the death of Clarence Madison Dally, one of his glassblowers. Dally had a habit of testing X-ray tubes on his hands, and acquired a cancerin them so tenacious that both arms wereamputated in a futile attempt to save his life.
The first use of X-rays under clinical conditions was by John Hall-Edwards inBirmingham, England on 11 January 1896, when he radiographed a needle stuck in the hand of an associate.[28] On 14 February 1896 Hall-Edwards was also the first to use X-rays in a surgical operation.[29] In early 1896, several weeks after Röntgen's discovery, Ivan Romanovich Tarkhanov irradiated frogs and insects with X-rays, concluding that the rays "not only photograph, but also affect the living function".[30]
The first medical X-ray made in the United States was obtained using a discharge tube of Pulyui's design. In January 1896, on reading of Röntgen's discovery, Frank Austin ofDartmouth College tested all of the discharge tubes in the physics laboratory and found that only the Pulyui tube produced X-rays. This was a result of Pulyui's inclusion of an oblique "target" of mica, used for holding samples offluorescent material, within the tube. On 3 February 1896 Gilman Frost, professor of medicine at the college, and his brother Edwin Frost, professor of physics, exposed the wrist of Eddie McCarthy, whom Gilman had treated some weeks earlier for a fracture, to the X-rays and collected the resulting image of the broken bone on gelatin photographic platesobtained from Howard Langill, a local photographer also interested in Röntgen's work.[31]
In 1901, U.S. President William McKinley was shot twice in an assassination attempt. While one bullet only grazed his sternum, another had lodged somewhere deep inside hisabdomen and could not be found. A worried McKinley aide sent word to inventor Thomas Edison to rush an X-ray machine to Buffalo to find the stray bullet. It arrived but wasn't used. While the shooting itself had not been lethal,gangrene had developed along the path of the bullet, and McKinley died of septic shock due to bacterial infection six days later.[32]
Hazards discovered
With the widespread experimentation with x‑rays after their discovery in 1895 by scientists, physicians, and inventors came many stories of burns, hair loss, and worse in technical journals of the time. In February 1896, Professor John Daniel and Dr. William Lofland Dudley of Vanderbilt Universityreported hair loss after Dr. Dudley was X-rayed. A child who had been shot in the head was brought to the Vanderbilt laboratory in 1896. Before trying to find the bullet an experiment was attempted, for which Dudley "with his characteristic devotion to science"[33][34][35] volunteered. Daniel reported that 21 days after taking a picture of Dudley'sskull (with an exposure time of one hour), he noticed a bald spot 2 inches (5.1 cm) in diameter on the part of his head nearest the X-ray tube: "A plate holder with the plates towards the side of the skull was fastened and a coin placed between the skull and the head. The tube was fastened at the other side at a distance of one-half inch from the hair."[36]
In August 1896 Dr. HD. Hawks, a graduate of Columbia College, suffered severe hand and chest burns from an x-ray demonstration. It was reported in Electrical Review and led to many other reports of problems associated with x-rays being sent in to the publication.[37]Many experimenters including Elihu Thomsonat Edison's lab, William J. Morton, and Nikola Tesla also reported burns. Elihu Thomson deliberately exposed a finger to an x-ray tube over a period of time and suffered pain, swelling, and blistering.[38] Other effects were sometimes blamed for the damage including ultraviolet rays and (according to Tesla) ozone.[39] Many physicians claimed there were no effects from x-ray exposure at all.[38]On August 3, 1905 at San Francisco,California, Elizabeth Fleischman, American woman X-ray pioneer, died from complications as a result of her work with X-rays.[40][41][42]
20th century and beyond

A patient being examined with a thoracic fluoroscope in 1940, which displayed continuous moving images. This image was used to argue thatradiation exposure during the X-ray procedure would be negligible.
The many applications of X-rays immediately generated enormous interest. Workshops began making specialized versions of Crookes tubes for generating X-rays and these first-generation cold cathode or Crookes X-ray tubes were used until about 1920.
Crookes tubes were unreliable. They had to contain a small quantity of gas (invariably air) as a current will not flow in such a tube if they are fully evacuated. However, as time passed, the X-rays caused the glass to absorb the gas, causing the tube to generate "harder" X-rays until it soon stopped operating. Larger and more frequently used tubes were provided with devices for restoring the air, known as "softeners". These often took the form of a small side tube which contained a small piece of mica, a mineral that traps relatively large quantities of air within its structure. A small electrical heater heated the mica, causing it to release a small amount of air, thus restoring the tube's efficiency. However, the mica had a limited life, and the restoration process was difficult to control.
In 1904, John Ambrose Fleming invented thethermionic diode, the first kind of vacuum tube. This used a hot cathode that caused anelectric current to flow in a vacuum. This idea was quickly applied to X-ray tubes, and hence heated-cathode X-ray tubes, called "Coolidge tubes", completely replaced the troublesome cold cathode tubes by about 1920.
In about 1906, the physicist Charles Barkladiscovered that X-rays could be scattered by gases, and that each element had a characteristic X-ray spectrum. He won the 1917 Nobel Prize in Physics for this discovery.
In 1912, Max von Laue, Paul Knipping, and Walter Friedrich first observed the diffractionof X-rays by crystals. This discovery, along with the early work of Paul Peter Ewald,William Henry Bragg, and William Lawrence Bragg, gave birth to the field of X-ray crystallography.
The Coolidge X-ray tube was invented during the following year by William D. Coolidge. It made possible the continuous emissions of X-rays. X-ray tubes similar to this are still in use in 2012.
The use of X-rays for medical purposes (which developed into the field of radiation therapy) was pioneered by Major John Hall-Edwards in Birmingham, England. Then in 1908, he had to have his left arm amputated because of the spread of X-ray dermatitis on his arm.[43]
From the 1920s through to the 1950s, x-ray machines were developed to assist in the fitting of shoes and were sold to commercial shoe stores.[44][45][46] Concerns regarding the impact of frequent or poorly controlled use were expressed in the 1950s,[47][48] leading to the practise's eventual end that decade.[49]
The Chandra X-ray Observatory, launched on July 23, 1999, has been allowing the exploration of the very violent processes in the universe which produce X-rays. Unlike visible light, which gives a relatively stable view of the universe, the X-ray universe is unstable. It features stars being torn apart byblack holes, galactic collisions, and novae, and neutron stars that build up layers of plasma that then explode into space.
An X-ray laser device was proposed as part of the Reagan Administration's Strategic Defense Initiative in the 1980s, but the only test of the device (a sort of laser "blaster" ordeath ray, powered by a thermonuclear explosion) gave inconclusive results. For technical and political reasons, the overall project (including the X-ray laser) was de-funded (though was later revived by the second Bush Administration as National Missile Defense using different technologies).
Phase-contrast X-ray imaging refers to a variety of techniques that use phase information of a coherent x-ray beam to image soft tissues. It has become an important method for visualizing cellular and histological structures in a wide range of biological and medical studies. There are several technologies being used for x-ray phase-contrast imaging, all utilizing different principles to convert phase variations in the x-rays emerging from an object into intensity variations.[50][51] These include propagation-based phase contrast,[52] talbotinterferometry,[51] refraction-enhanced imaging,[53] and x-ray interferometry.[54] These methods provide higher contrast compared to normal absorption-contrast x-ray imaging, making it possible to see smaller details. A disadvantage is that these methods require more sophisticated equipment, such assynchrotron or microfocus x-ray sources, X-ray optics, and high resolution x-ray detectors.
Energy ranges
Soft and hard X-rays
X-rays with high photon energies (above 5–10 keV, below 0.2–0.1 nm wavelength) are calledhard X-rays, while those with lower energy are called soft X-rays.[55] Due to their penetrating ability, hard X-rays are widely used to image the inside of objects, e.g., in medical radiography and airport security. The term X-ray is metonymically used to refer to aradiographic image produced using this method, in addition to the method itself. Since the wavelengths of hard X-rays are similar to the size of atoms they are also useful for determining crystal structures by X-ray crystallography. By contrast, soft X-rays are easily absorbed in air; the attenuation lengthof 600 eV (~2 nm) X-rays in water is less than 1 micrometer.[56]
Gamma rays
There is no consensus for a definition distinguishing between X-rays and gamma rays. One common practice is to distinguish between the two types of radiation based on their source: X-rays are emitted by electrons, while gamma rays are emitted by the atomic nucleus.[57][58][59][60] This definition has several problems: other processes also can generate these high-energy photons, or sometimes the method of generation is not known. One common alternative is to distinguish X- and gamma radiation on the basis of wavelength (or, equivalently, frequency or photon energy), with radiation shorter than some arbitrary wavelength, such as 10−11 m (0.1 Å), defined as gamma radiation.[61] This criterion assigns a photon to an unambiguous category, but is only possible if wavelength is known. (Some measurement techniques do not distinguish between detected wavelengths.) However, these two definitions often coincide since the electromagnetic radiation emitted by X-ray tubes generally has a longer wavelength and lower photon energy than the radiation emitted by radioactive nuclei.[57] Occasionally, one term or the other is used in specific contexts due to historical precedent, based on measurement (detection) technique, or based on their intended use rather than their wavelength or source. Thus, gamma-rays generated for medical and industrial uses, for example radiotherapy, in the ranges of 6–20MeV, can in this context also be referred to as X-rays.[citation needed]
Properties
X-ray photons carry enough energy to ionizeatoms and disrupt molecular bonds. This makes it a type of ionizing radiation, and therefore harmful to living tissue. A very highradiation dose over a short period of time causes radiation sickness, while lower doses can give an increased risk of radiation-induced cancer. In medical imaging this increased cancer risk is generally greatly outweighed by the benefits of the examination. The ionizing capability of X-rays can be utilized in cancer treatment to killmalignant cells using radiation therapy. It is also used for material characterization usingX-ray spectroscopy.
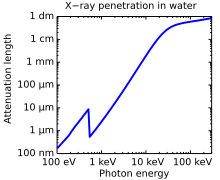
Attenuation length of X-rays in water showing the oxygen absorption edgeat 540 eV, the energy−3 dependence ofphotoabsorption, as well as a leveling off at higher photon energies due toCompton scattering. The attenuation length is about four orders of magnitude longer for hard X-rays (right half) compared to soft X-rays (left half).
Hard X-rays can traverse relatively thick objects without being much absorbed orscattered. For this reason, X-rays are widely used to image the inside of visually opaque objects. The most often seen applications are in medical radiography and airport securityscanners, but similar techniques are also important in industry (e.g. industrial radiography and industrial CT scanning) and research (e.g. small animal CT). Thepenetration depth varies with several orders of magnitude over the X-ray spectrum. This allows the photon energy to be adjusted for the application so as to give sufficienttransmission through the object and at the same time provide good contrast in the image.
X-rays have much shorter wavelengths than visible light, which makes it possible to probe structures much smaller than can be seen using a normal microscope. This property is used in X-ray microscopy to acquire high resolution images, and also in X-ray crystallography to determine the positions ofatoms in crystals.
Interaction with matter
X-rays interact with matter in three main ways, through photoabsorption, Compton scattering, and Rayleigh scattering. The strength of these interactions depends on the energy of the X-rays and the elemental composition of the material, but not much on chemical properties, since the X-ray photon energy is much higher than chemical binding energies. Photoabsorption or photoelectric absorption is the dominant interaction mechanism in the soft X-ray regime and for the lower hard X-ray energies. At higher energies, Compton scattering dominates.
Photoelectric absorption
The probability of a photoelectric absorption per unit mass is approximately proportional toZ3/E3, where Z is the atomic number and E is the energy of the incident photon.[62] This rule is not valid close to inner shell electron binding energies where there are abrupt changes in interaction probability, so calledabsorption edges. However, the general trend of high absorption coefficients and thus shortpenetration depths for low photon energies and high atomic numbers is very strong. For soft tissue, photoabsorption dominates up to about 26 keV photon energy where Compton scattering takes over. For higher atomic number substances this limit is higher. The high amount of calcium (Z=20) in bones together with their high density is what makes them show up so clearly on medical radiographs.
A photoabsorbed photon transfers all its energy to the electron with which it interacts, thus ionizing the atom to which the electron was bound and producing a photoelectron that is likely to ionize more atoms in its path. An outer electron will fill the vacant electron position and produce either a characteristic photon[clarification needed] or an Auger electron. These effects can be used for elemental detection through X-ray spectroscopy orAuger electron spectroscopy.
Compton scattering
Compton scattering is the predominant interaction between X-rays and soft tissue in medical imaging.[63] Compton scattering is aninelastic scattering of the X-ray photon by an outer shell electron. Part of the energy of the photon is transferred to the scattering electron, thereby ionizing the atom and increasing the wavelength of the X-ray. The scattered photon can go in any direction, but a direction similar to the original direction is more likely, especially for high-energy X-rays. The probability for different scattering angles are described by the Klein–Nishina formula. The transferred energy can be directly obtained from the scattering angle from theconservation of energy and momentum.
Rayleigh scattering
Rayleigh scattering is the dominant elastic scattering mechanism in the X-ray regime.[64]Inelastic forward scattering gives rise to the refractive index, which for X-rays is only slightly below 1.[65]
Production
Whenever charged particles (electrons or ions) of sufficient energy hit a material, X-rays are produced.
Production by electrons

Spectrum of the X-rays emitted by an X-ray tube with a rhodium target, operated at 60 kV. The smooth, continuous curve is due tobremsstrahlung, and the spikes arecharacteristic K lines for rhodium atoms.
X-rays can be generated by an X-ray tube, avacuum tube that uses a high voltage to accelerate the electrons released by a hot cathode to a high velocity. The high velocity electrons collide with a metal target, theanode, creating the X-rays.[68] In medical X-ray tubes the target is usually tungsten or a more crack-resistant alloy of rhenium (5%) and tungsten (95%), but sometimes molybdenumfor more specialized applications, such as when softer X-rays are needed as in mammography. In crystallography, a coppertarget is most common, with cobalt often being used when fluorescence from ironcontent in the sample might otherwise present a problem.
The maximum energy of the produced X-rayphoton is limited by the energy of the incident electron, which is equal to the voltage on the tube times the electron charge, so an 80 kV tube cannot create X-rays with an energy greater than 80 keV. When the electrons hit the target, X-rays are created by two different atomic processes:
- Characteristic X-ray emission (X-ray fluorescence): If the electron has enough energy it can knock an orbital electron out of the inner electron shell of a metal atom, and as a result electrons from higher energy levels then fill up the vacancy and X-ray photons are emitted. This process produces an emission spectrum of X-rays at a few discrete frequencies, sometimes referred to as the spectral lines. The spectral lines generated depend on the target (anode) element used and thus are called characteristic lines. Usually these are transitions from upper shells into K shell (called K lines), into L shell (called L lines) and so on.
- Bremsstrahlung: This is radiation given off by the electrons as they are scattered by the strong electric field near the high-Z (protonnumber) nuclei. These X-rays have acontinuous spectrum. The intensity of the X-rays increases linearly with decreasing frequency, from zero at the energy of the incident electrons, the voltage on the X-ray tube.
So the resulting output of a tube consists of a continuous bremsstrahlung spectrum falling off to zero at the tube voltage, plus several spikes at the characteristic lines. The voltages used in diagnostic X-ray tubes range from roughly 20 kV to 150 kV and thus the highest energies of the X-ray photons range from roughly 20 keV to 150 keV.[69]
Both of these X-ray production processes are inefficient, with a production efficiency of only about one percent, and thus most of theelectric power consumed by the tube is released as waste heat. When producing a usable flux of X-rays, the X-ray tube must be designed to dissipate the excess heat.
Short nanosecond bursts of X-rays peaking at 15-keV in energy may be reliably produced by peeling pressure-sensitive adhesive tape from its backing in a moderate vacuum. This is likely to be the result of recombination of electrical charges produced by triboelectric charging. The intensity of X-raytriboluminescence is sufficient for it to be used as a source for X-ray imaging.[70]
A specialized source of X-rays which is becoming widely used in research issynchrotron radiation, which is generated byparticle accelerators. Its unique features are X-ray outputs many orders of magnitude greater than those of X-ray tubes, wide X-ray spectra, excellent collimation, and linear polarization.[71]
Production by fast positive ions
X-rays can also be produced by fast protons or other positive ions. The proton-induced X-ray emission or particle-induced X-ray emission is widely used as an analytical procedure. For high energies, the productioncross section is proportional to Z12Z2−4, whereZ1 refers to the atomic number of the ion, Z2to that of the target atom.[72] An overview of these cross sections is given in the same reference.
Detectors
X-ray detectors vary in shape and function depending on their purpose. Imaging detectors such as those used for radiographywere originally based on photographic platesand later photographic film, but are now mostly replaced by various digital detector types such as image plates and flat panel detectors. For radiation protection direct exposure hazard is often evaluated usingionization chambers, while dosimeters are used to measure the radiation dose a person has been exposed to. X-ray spectra can be measured either by energy dispersive or wavelength dispersive spectrometers.
Medical uses
Since Röntgen's discovery that X-rays can identify bone structures, X-rays have been used for medical imaging. The first medical use was less than a month after his paper on the subject.[31] Up to 2010, 5 billion medical imaging examinations had been conducted worldwide.[73] Radiation exposure from medical imaging in 2006 made up about 50% of total ionizing radiation exposure in the United States.[74]
Projectional radiographs
Projectional radiography is the practice of producing two-dimensional images using x-ray radiation. Bones contain much calcium, which due to its relatively high atomic numberabsorbs x-rays efficiently. This reduces the amount of X-rays reaching the detector in the shadow of the bones, making them clearly visible on the radiograph. The lungs and trapped gas also show up clearly because of lower absorption compared to tissue, while differences between tissue types are harder to see.
Projectional radiographs are useful in the detection of pathology of the skeletal systemas well as for detecting some disease processes in soft tissue. Some notable examples are the very common chest X-ray, which can be used to identify lung diseases such as pneumonia, lung cancer, orpulmonary edema, and the abdominal x-ray, which can detect bowel (or intestinal) obstruction, free air (from visceral perforations) and free fluid (in ascites). X-rays may also be used to detect pathology such asgallstones (which are rarely radiopaque) orkidney stones which are often (but not always) visible. Traditional plain X-rays are less useful in the imaging of soft tissues such as the brain or muscle.
In medical diagnostic applications, the low energy (soft) X-rays are unwanted, since they are totally absorbed by the body, increasing the radiation dose without contributing to the image. Hence, a thin metal sheet, often ofaluminium, called an X-ray filter, is usually placed over the window of the X-ray tube, absorbing the low energy part in the spectrum. This is called hardening the beam since it shifts the center of the spectrum towards higher energy (or harder) x-rays.
To generate an image of the cardiovascular system, including the arteries and veins (angiography) an initial image is taken of the anatomical region of interest. A second image is then taken of the same region after an iodinated contrast agent has been injected into the blood vessels within this area. These two images are then digitally subtracted, leaving an image of only the iodinated contrast outlining the blood vessels. Theradiologist or surgeon then compares the image obtained to normal anatomical images to determine whether there is any damage or blockage of the vessel.
Computed tomography
Computed tomography (CT scanning) is a medical imaging modality where tomographic images or slices of specific areas of the body are obtained from a large series of two-dimensional X-ray images taken in different directions.[75] These cross-sectional images can be combined into a three-dimensionalimage of the inside of the body and used for diagnostic and therapeutic purposes in various medical disciplines.
Fluoroscopy
Fluoroscopy is an imaging technique commonly used by physicians or radiation therapists to obtain real-time moving images of the internal structures of a patient through the use of a fluoroscope. In its simplest form, a fluoroscope consists of an X-ray source and a fluorescent screen, between which a patient is placed. However, modern fluoroscopes couple the screen to an X-ray image intensifier and CCD video camera allowing the images to be recorded and played on a monitor. This method may use a contrast material. Examples include cardiac catheterization (to examine for coronary artery blockages) and barium swallow (to examine for esophageal disorders).
Radiotherapy
The use of X-rays as a treatment is known asradiation therapy and is largely used for the management (including palliation) of cancer; it requires higher radiation doses than those received for imaging alone. X-rays beams are used for treating skin cancers using lower energy x-ray beams while higher energy beams are used for treating cancers within the body such as brain, lung, prostate, and breast.[76][77]
Adverse effects

Abdominal radiograph of a pregnant woman, a procedure that should be performed only after proper assessment of benefit versus risk
Diagnostic X-rays (primarily from CT scans due to the large dose used) increase the risk of developmental problems and cancer in those exposed.[78][79][80] X-rays are classified as a carcinogen by both the World Health Organization's International Agency for Research on Cancer and the U.S. government.[73][81] It is estimated that 0.4% of current cancers in the United States are due to computed tomography (CT scans) performed in the past and that this may increase to as high as 1.5-2% with 2007 rates of CT usage.[82]
Experimental and epidemiological data currently do not support the proposition that there is a threshold dose of radiation below which there is no increased risk of cancer.[83]However, this is under increasing doubt.[84] It is estimated that the additional radiation will increase a person's cumulative risk of getting cancer by age 75 by 0.6–1.8%.[85] The amount of absorbed radiation depends upon the type of X-ray test and the body part involved.[86] CT and fluoroscopy entail higher doses of radiation than do plain X-rays.
To place the increased risk in perspective, a plain chest X-ray will expose a person to the same amount from background radiation that people are exposed to (depending upon location) every day over 10 days, while exposure from a dental X-ray is approximately equivalent to 1 day of environmental background radiation.[87] Each such X-ray would add less than 1 per 1,000,000 to the lifetime cancer risk. An abdominal or chest CT would be the equivalent to 2–3 years of background radiation to the whole body, or 4–5 years to the abdomen or chest, increasing the lifetime cancer risk between 1 per 1,000 to 1 per 10,000.[87] This is compared to the roughly 40% chance of a US citizen developing cancer during their lifetime.[88] For instance, the effective dose to the torso from a CT scan of the chest is about 5 mSv, and the absorbed dose is about 14 mGy.[89] A head CT scan (1.5mSv, 64mGy)[90] that is performed once with and once without contrast agent, would be equivalent to 40 years of background radiation to the head. Accurate estimation of effective doses due to CT is difficult with the estimation uncertainty range of about ±19% to ±32% for adult head scans depending upon the method used.[91]
The risk of radiation is greater to a fetus, so in pregnant patients, the benefits of the investigation (X-ray) should be balanced with the potential hazards to the fetus.[92][93] In the US, there are an estimated 62 million CT scans performed annually, including more than 4 million on children.[86] Avoiding unnecessary X-rays (especially CT scans) reduces radiation dose and any associated cancer risk.[94]
Medical X-rays are a significant source of man-made radiation exposure. In 1987, they accounted for 58% of exposure from man-made sources in the United States. Since man-made sources accounted for only 18% of the total radiation exposure, most of which came from natural sources (82%), medical X-rays only accounted for 10% of total American radiation exposure; medical procedures as a whole (including nuclear medicine) accounted for 14% of total radiation exposure. By 2006, however, medical procedures in the United States were contributing much more ionizing radiation than was the case in the early 1980s. In 2006, medical exposure constituted nearly half of the total radiation exposure of the U.S. population from all sources. The increase is traceable to the growth in the use of medical imaging procedures, in particularcomputed tomography (CT), and to the growth in the use of nuclear medicine.[74][95]
Dosage due to dental X-rays varies significantly depending on the procedure and the technology (film or digital). Depending on the procedure and the technology, a single dental X-ray of a human results in an exposure of 0.5 to 4 mrem. A full mouth series of X-rays may result in an exposure of up to 6 (digital) to 18 (film) mrem, for a yearly average of up to 40 mrem.









Comments
Post a Comment